Innovation
The Newcastle Microsatellite Instability-Plus assay for high throughput Lynch syndrome screening
Competition
NHS Cancer Programme – Innovation Open Call 1
Name and role of Project Lead
Dr Ciaron McAnulty, Deputy Head of Laboratory, (Rare Disease Lead, Newcastle Genetics Laboratory), The Newcastle Upon Tyne Hospitals NHS Foundation Trust
Team Twitter feeds: @GeordieGenomes @CaPP3
Clinical Problem
Lynch syndrome (LS) affects ~3/1,000 people. LS carriers often get bowel and other cancers from early adulthood; by age 65 years more than two thirds will have had at least one cancer. LS cancers can be prevented or detected early, but only ~5% of carriers are known to the NHS. In 2017, NICE required all colorectal cancers (CRCs) to be tested for Mismatch Repair (MMR) defects to help identify people with
LS. Implementation remains a work in progress: A national audit of NHS data from 2019 found only 22% of CRCs completed testing, meaning an estimated ~700 LS diagnoses were missed.
Proposed Solution
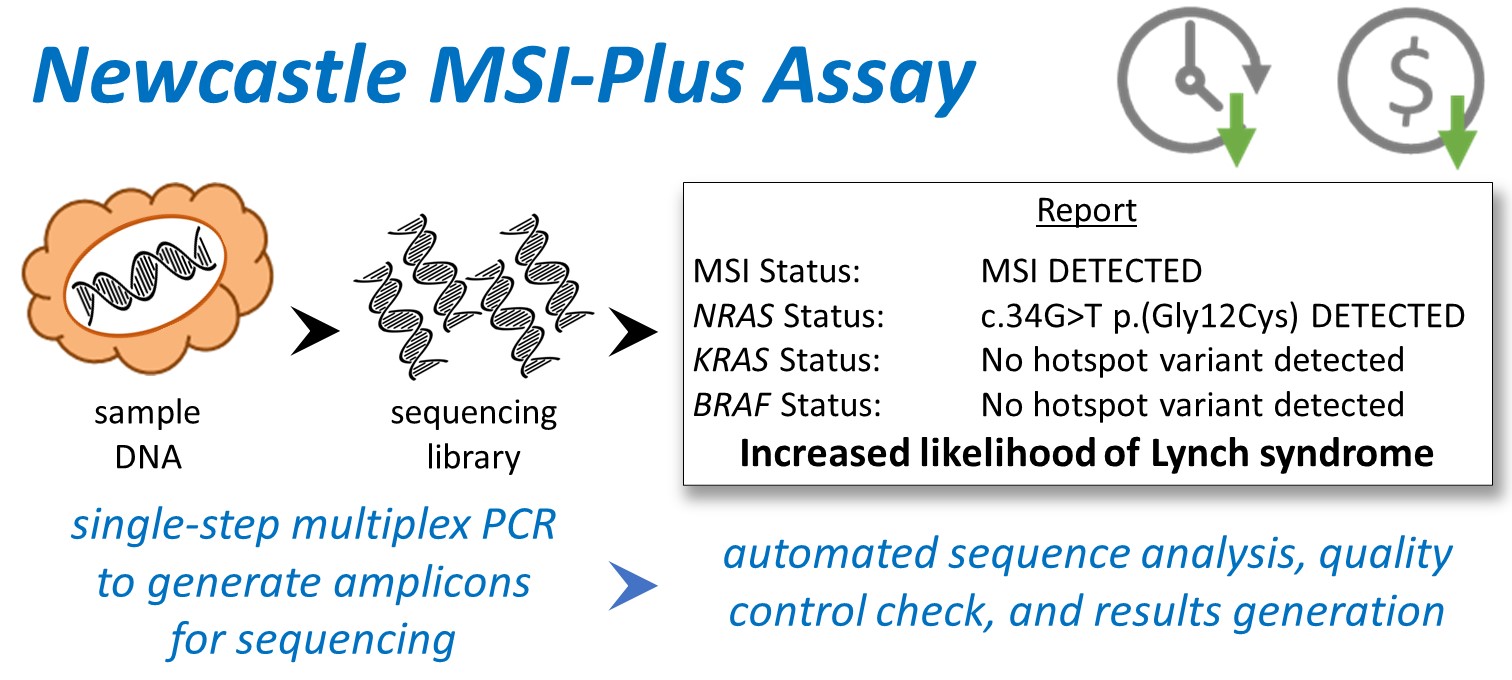
One in six CRCs are MMR deficient. MMR deficiency can be detected by testing for microsatellite instability (MSI) - mutations in repetitive DNA sequences called microsatellites. Approximately 20% of MMR deficient CRCs are caused by LS. LS CRCs lack the driver mutation BRAF V600E that is found in half of the sporadic cases. Currently, MSI and BRAF V600E are tested separately to screen for LS. The Newcastle MSIPlus assay combines analysis of novel MSI markers and BRAF/RAS driver mutations in a single tumour test using next generation sequencing. The multiplex polymerase chain reaction approach allows rapid preparation of sequencing libraries from ~1 nanogram of DNA. Custom analysis software presents a clear YES/NO result on referral for germline testing, allowing reporting at scale without senior scientist/pathologist involvement. A single technologist can analyse and report on all CRCs across 3 million people for high volume and low-cost LS screening, making the service more efficient and effective.
People being treated for cancer will be approached for a blood sample for germline testing while still under hospital care rather than being approached long after discharge or lost to follow-up. Thousands of LS families at risk of recurrent cancers and early deaths will also be offered effective surveillance and simple proven prevention using regular aspirin.
Market Traction and Implementation
-
Cancer Research UK manage the Intellectual Property of the MSI-Plus assay for Newcastle University and has licensed the assay to Newcastle Hospitals. Income will derive from service provision via the Genomic Test Directory. The assay will be validated for IVDR and FDA 510K for sale in other health systems.
-
The assay is being validated across all tumour types to facilitate use of Immune Checkpoint
Inhibitors and has been shown to effectively identify urothelial cancers as a “urine liquid biopsy”- a research evaluation of the assay as a routine postal screening service for urinary tract and endometrial cancers for LS carriers is underway. -
The assay is currently implemented in: The Newcastle upon Tyne Hospitals NHS Foundation Trust, Northumbria Healthcare NHS Foundation Trust, Gateshead NHS Foundation Trust, South Tyneside and Sunderland NHS Foundation Trust, County Durham and Darlington NHS Foundation Trust, South Tees Hospitals NHS Foundation Trust, North Tees and Hartlepool NHS Foundation Trust, and North Cumbria Integrated care NHS Foundation Trust.
Impact - Early detection and diagnosis of cancer
• We have now replaced expensive, time-consuming immunohistochemistry screening in all eight hospitals in the North East and Cumbria with MSIPlus; a cheap simple “one stop” analysis of CRC biopsies with a seven day turnaround, enhancing the search for LS cases. Identified LS carriers will benefit from cancer prevention and surveillance.
• SBRI Healthcare have supported further roll out of the assay to the rest of our North East and Yorkshire Genomic Laboratory Hub (GLH) and to four of the other six GLHs in England.
• Around a third of CRCs have BRAF V600E or another Ras/MAPK driver mutation and 1 in 6 are MMR deficient. Timely testing by MSI-Plus will enhance therapy by targeting appropriate drugs such as the new highly effective Immune Checkpoint Inhibitors.
Impact - patient outcomes and experience
- The LS community is fully engaged and supporting further research to effectively treat, detect and prevent cancer.
Impact - Service delivery
-
The North East Region’s pathology community has agreed to replace immunohistochemistry with the MSI-Plus assay as the first line tumour screening approach, cutting costs and reducing pressure on pathology services.
-
More than 5,000 tests have been reported in the North East and Cumbria with an increase from well below 30% to close to 100% completion of the tumour screening pathway. There has been a reduction in the loss of patients between the tumour test and the offer of a blood test thanks to the 7 day turnaround. Other parts of England will soon begin to use the assay at scale once the current UKAS assessment is complete.
-
An independent review by the Academic Health Science Network showed strong professional support. Multidisciplinary teams now routinely ask for the MSI-Plus result. A formal economic analysis by Newcastle University is underway.
Clinician quote
"As a GI pathologist, the availability of MSI-Plus technology has made testing for MSI easy and straightforward to use. Feedback from clinical users namely oncologists and surgeons has been complimentary, in particular, as a one-stop shop which includes Lynch screening function, triaging oncological management with the MSI/RAS status as well as the simplicity and clarity of reports. From a management perspective as the Lynch Champion for North East & North Cumbria region, MSI-Plus has made it possible to streamline and optimise the Lynch detection services in a relatively large scale which covers a population of over 2,000 new colorectal cancer patients annually in 8 separate NHS Foundation Trusts."
Dr Peh-Sun Loo, Consultant Histopathologist, The Newcastle upon Tyne Hospitals NHS Foundation Trust
Date Published
September 2023